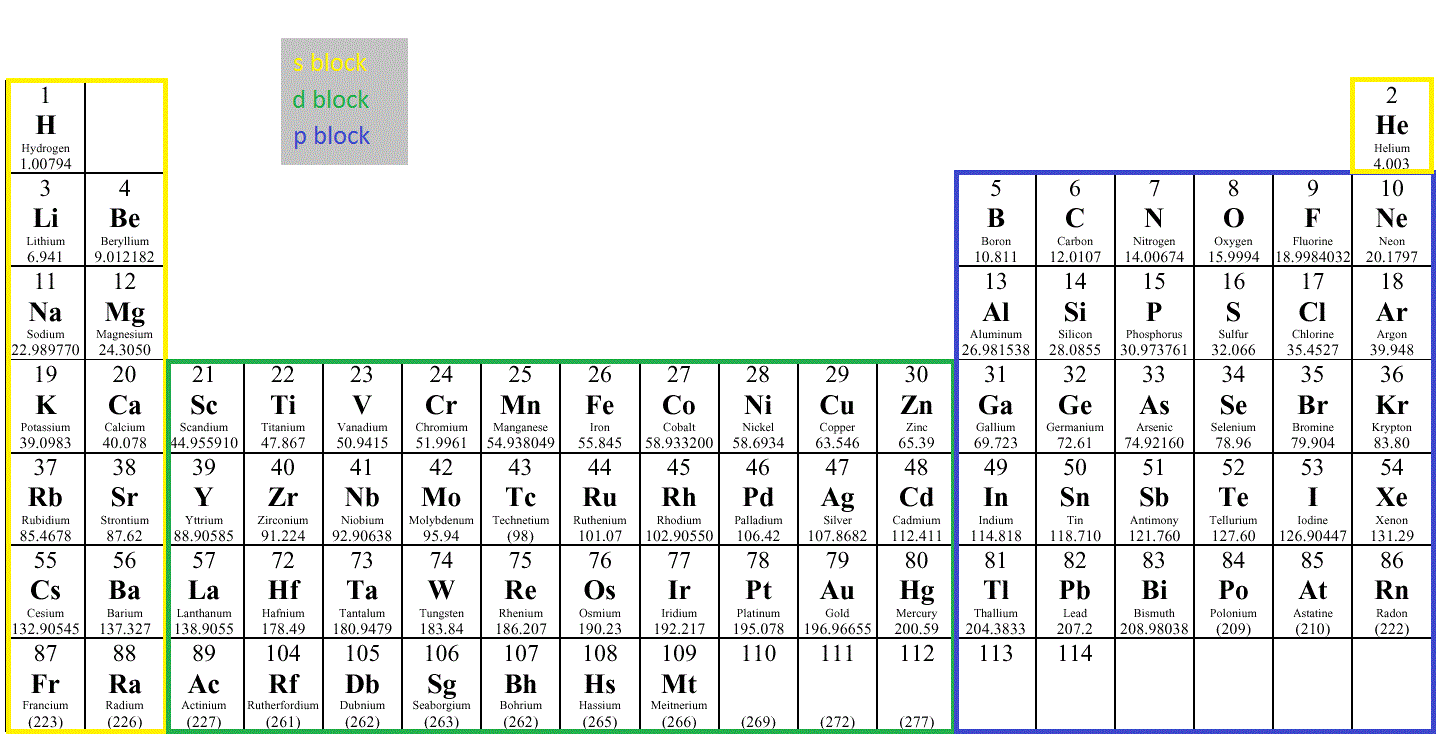
Each element has a different number of electrons, and therefore a different electronic configuration. Electronic configurations tell us the number of electrons in an atom, and where the electrons are, and often even the properties of the element, as electronic configurations correspond to the Periodic Table.
In a configuration, the large number at the front is the shell number, the letter is the name of the subshell, the small letter at the bottom (sometimes shown on p subshells as x, y & or z) is the orbital, and the small number at the top is the number of electrons in the subshell or orbital.
For example, Magnesium (Mg) has the electronic configuration: 1s22s22p63s2
From this information, we can see that Magnesium has 12 electrons. We know from the previous page that s sub-shells can only hold 2 electrons, and that p sub-shells can hold a maximum of 6 electrons, so we know that all of the shells are full. The last subshell tells us that Magnesium is 3 down in the s block, and two across as it contains 2 electrons. (Group 2 metal) These two electrons would be easy to lose in a reaction, so would be fairly reactive &,which is why the Magnesium ion is 2+.
If you are asked to give the electronic configuration for an element, for example Fluorine, you may use either of two ways:
You can use the periodic table, and go along the blocks until you reach the element you are looking for. The first period / row across contains only Hydrogen & Helium & so is 1s2. The second row contains Lithium & Beryllium in the p-block ( 2s2) & Fluorine is 5 elements along in the p block on the 2nd row, so will be 2p5.
Therefore, the configuration is 1s22s22p5.
If you do not have a periodic table to hand, you may also work it out using rules, if you know how many electrons are present.
1s is filled first, then 2s, then 2p, then 3s, then 3p. Pretty straightforward. Then comes the tricky part. After 3p, we move to 4s as we usually would, but then to 3d before moving to 4p. Then 5s, 4d, then 5p. Have a look at the Periodic Table above to help your understanding.